
| |
| Home |
| About Us |
| Services |
| Contact Info |
| Services |
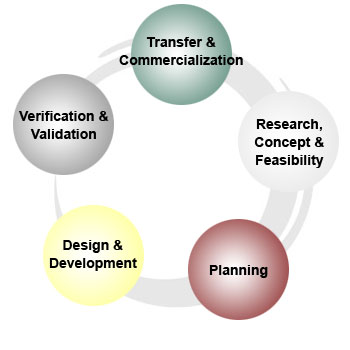 Canopy Medical is a consulting firm that provides strategic product development services including regulatory, clinical, quality, product development, business development and operations throughout the product life cycle. |
Research, Concept & Feasibility
|
Planning
|
Design and Development
|
Verification and Validation
|
Transfer and Commercialization
|